As people, we have daily recommended values of nutrients in order for us to live healthily. While we don’t have an FDA for plants, we understand which nutrients and in what combination various types of plants need to thrive.
Many gardeners only start thinking these things when they notice signs of stress in their plants and start wondering about the root causes of yellowing leaves, weak stems, or stunted growth.
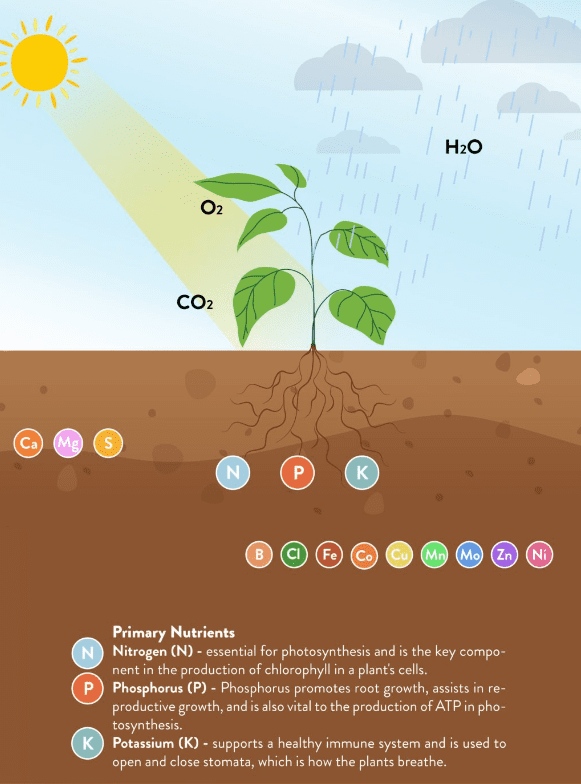
For optimal plant health, plants require 18 essential nutrients. These can be broken down into three plant nutrient categories: 1) macronutrients, 2) secondary nutrients, and 3) micronutrients.
Primary macronutrients
These are the 3 macronutrients that plants require in the largest quantities for health and growth, they are nitrogen (N), phosphorus (P), and potassium (K).
Plants combine these primate macronutrients with sunlight, air, and water to make their own food through the process of photosynthesis.
You’ll often see plant fertilizer and plant food displaying an NPK ratio on their label, which speaks to their ratio of nitrogen, phosphorus, and potassium. For example, if it reads 9-3-6, by weight the fertilizer contains 9% N, 3% P, and 6% K. The rest of the weight is taken up by various micronutrients, water, and fillers.
In the context of fertilizers, there are also 3 other essential macronutrients that aren’t talked about: carbon (C), hydrogen (H), and oxygen (O).
The reason you won’t hear these nutrients mentioned is pretty simple. There is an abundance of these elements in our environment. Meaning, that in normal growing conditions, there is no need to supplement them with some form of fertilizer.
Although, this doesn’t mean they aren’t important and, in fact, plants will experience a lack of these elements – ultimately leading to deficiencies.
Here’s a breakdown of these primary nutrients.
Nitrogen (N)
Where it comes from: Nitrogen can be obtained through natural sources like decaying organic matter, minerals, and atmospheric gasses (N2).
How it’s absorbed: Thanks to two different types of bacteria that fixate and convert nitrogen into fixed nitrogen, plants can absorb nitrogen through their roots for various metabolic requirements.
Why it’s important: As an essential nutrient, nitrogen is one of the most important macronutrients for plants. It facilitates photosynthesis and is the key component in the production of chlorophyll in a plant’s cells.
Nitrogen is also integral for the product of essential amino acids and proteins that are used for various metabolic processes that are vital for a healthy, growing plant.
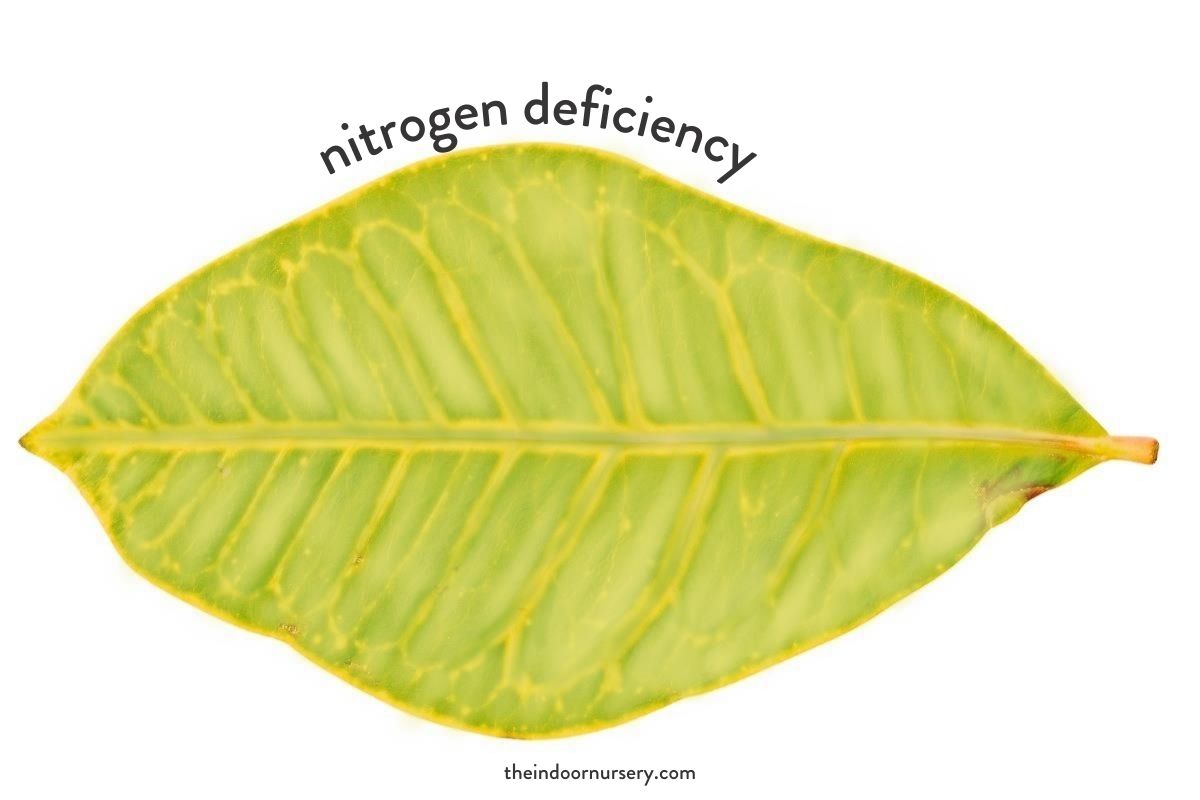
Signs of deficiency: The signs of nitrogen deficiency in plants are stunted growth, a pale green coloration that covers the entire leaf, and early senescence (death) of mature or older leaves.
Phosphorus (P)

Where it comes from: Commonly in the form of phosphate salts, most phosphorus that is used by plants originates from ocean sediments or rock formations. Overtime and with weathering, the phosphate salts predominantly dissolve into groundwater that then finds its way to plants.
Decomposing and decaying organic matter is also very high in phosphorus and is often used by gardeners to promote root growth and winter hardiness.
How it’s absorbed: As most phosphorus is found in groundwater, phosphorus is readily available to plants as phosphate and is absorbed by plants through their root systems.
Why it’s important: Phosphorus is another important macronutrient in terms of photosynthesis, health, and vigor. Phosphorus promotes root growth, assists in reproductive growth, and is also vital to the production of ATP – plants’ “energy units” that form during photosynthesis.
Phosphorus is also essential for the development of plant roots, flowers, seeds, and fruit.
Signs of deficiency: The most obvious signs of a phosphorus deficiency is a reddish/purplish coloration presenting itself on both a leaf’s veins or margins. Phosphorus deficiencies also limit root growth, cause stunted shoots, and decreased leaf size.
Plants with a phosphorus deficiency will also produce smaller seeds, experience delayed maturity, and have a higher susceptibility to disease.
Potassium (K)

Where it comes from: When a plant uses potassium, it is taking it from the soil’s mineral content. The mineral content of soil is determined by the bedrock and sedimentary rocks in an area, and any other materials like animal droppings or human waste that wash down from higher elevations.
How it’s absorbed: Plants need potassium to make enzymes and other organic compounds that carry out various metabolic processes.
Why it’s important: Potassium is key in creating a healthy immune system for your plants. It helps to promote overall plant health, also safeguarding them from pests and disease. Potassium is also used by plants to both open and close their stomata. Without it, their stomata would stay open or close, leaving them with no method of gas exchange.
Signs of deficiency: Potassium deficiency is characterized by pale, yellow leaves. Unlike a nitrogen deficiency that discolors the entire leaf, the coloration that indicates a potassium deficiency starts at the leaf margins and bleeds towards the center, most times leaving a green center.
Symptoms of potassium deficiency in plants also include wilting, early leaf fall, and loss of turgidity. Turbidity occurs when plant cells are stiff and filled with water; drooping leaves are typical of a potassium deficient plant.
Carbon (C)

Where it comes from: Carbon exists in the ground as organic matter and as carbon dioxide in the atmosphere. Plants chemically process carbon, releasing CO2 and H2O gasses. When plants are deprived of these carbon-containing compounds, their growth rate slows.
As I’m sure you’re well aware of, there certainly isn’t a shortage of carbon in our atmosphere at the moment – in fact, it’s quite the opposite. So a carbon deficiency isn’t an issue you’re likely to come across. Nevertheless, I’d be remiss if I didn’t include it, as carbon is absolutely essential to the health of your houseplants.
How it’s absorbed: Plants absorb carbon from both the soil using their roots and from the atmosphere through photosynthesis. During photosynthesis, plants use energy from sunlight to produce glucose from carbon dioxide and water.
This process needs chlorophyll, a series of molecules that give plant leaves their green pigmentation. Oxygen is released during this process, which, of course, supports all other life forms on earth.
Why it’s important: Aside from creating sugars and plant energy during photosynthesis, carbon is also vital for making essential amino acids, proteins, and nucleic acids, which are important molecules in cell function, growth, and reproduction.
Signs of deficiency: When plants are starved of carbon dioxide, their predominant source of carbon, they cannot photosynthesize efficiently. This stunts their growth and overall vigor.
Hydrogen (H)

Where it comes from: The hydrogen that plants use is most often derived from water. In the sunlit areas of the plant, water undergoes a series of reactions which produce oxygen and hydrogen ions.
How it’s absorbed: In photosynthesis, water is broken down by the electron transport chain to provide the energy needed for the plant’s synthesis of glucose. Glucose is then converted into a form called pyruvate by the process of glycolysis.
Why it’s important: Hydrogen is a very important element as plant cells use hydrogen to convert sunlight into energy through photosynthesis. Plants also release oxygen during this process. The water cycles back into the atmosphere and supports other living organisms.
Hydrogen also plays an integral role in soil pH. If the soil pH is too low or too high, caused by acidic soils or alkaline soils, nutrient lockout occurs. Basically, nutrient lockout is the inability of a plant’s root system to absorb nutrients and minerals from the soil as easily as they should be able to.
Signs of deficiency: As plants use water as their source of hydrogen, signs of a deficiency will be very obvious. They will look dehydrated or exhibit nutrient deficiencies caused by nutrient lockout.
Oxygen (O)

Where it comes from: Oxygen that plants use comes from the air and the soil. Oxygen is both stored for energy and released as a byproduct into the atmosphere.
How it’s absorbed: All plant cells are constantly respiring, meaning they constantly need oxygen. Therefore, plants absorb oxygen both through their roots and their leaves. The leaves create their own oxygen while photosynthesizing, while the roots absorb oxygen that is locked between soil particles.
Why it’s important: In the process of photosynthesis, the plant will combine carbon dioxide and water with light from the sun to produce glucose and oxygen. Without enough oxygen, plants cannot produce glucose or undergo cellular respiration.
Signs of deficiency: Most times there is an abundance of oxygen surrounding a plant’s leaves, but underground is a different story. If a growing medium, like soil, gets too compact, or a plant is over-watered, this will cause anaerobic conditions. This results in a lack of air being contained between soil particles and ultimately will starve the root system of oxygen.
Even though hydroponic and semi-hydroponic plants require different nutrients than those grown in soil, they will display similar signs of distress when starved for oxygen. Whether you have a DIY hydroponic system or a professional setup, your plant’s roots can get starved for oxygen if you don’t use a bubbler or change the water frequently enough.
If this occurs, a plant will wilt, distort, and have a lack of vigor. Both young and older leaves will also drop prematurely and show signs of chlorosis (yellowing) and necrosis (die-off). Left to the extreme, a saturated or over-watered plant will experience root rot and, if left untreated, will die.
Secondary plant nutrients
Calcium, magnesium, and sulfur are all essential to plant nutrients. These 3 elements are referred to as “secondary” nutrients, as plants require them in smaller quantities than macronutrients but in larger quantities than micronutrients.
Calcium (Ca)

Where it comes from: Parent material, made up of geologic reservoirs, constantly replenishes healthy soils of calcium.
Calcium also has formed over millions of years from the deposition of biogenic calcium carbonate, or rather from the buildup of marine organism shells collecting at the bottom of the world’s oceans. This depositing layer over time cements and forms limestone, the dominant source of the world’s calcium.
How it’s absorbed: Plants derive calcium from the soil through their root systems, where solutes are taken up by osmosis. Root growth is stimulated by calcium ions in the rhizosphere (the area between the root tip and the soil).
Why it’s important: Calcium is a nutrient that is important for many processes in a plant’s cells, including the transport of nutrients and the storage and release of energy. Strangely, evidence presented by NASA suggests that root uptake of calcium is necessary for a plant to sense gravity.
How cool is that?!
Signs of deficiency: Plants suffering from a calcium deficiency will display yellowing, particularly around the edge of the leaf. Younger leaves rarely form properly, remain small, and will often curl at the edges.
A calcium deficiency can also lead to stunted growth, poor fruit quality, and frail looking flowers.
Magnesium (Mg)

Where it comes from: One of the most abundant mineral nutrients in the Earth’s crust is magnesium. It accounts for about 2% of its mass and is found in large quantities in limestone, sandstone, and marble. The magnesium that is used by plants most commonly comes from weathering of rocks that, over time, leach minerals into soil.
How it’s absorbed: Plants derive magnesium from – you guessed it – the soil. It is taken in through the roots, then has to be transported to different areas of the plant for functions such as chlorophyll production and photosynthesis.
Why it’s important: Plants need magnesium to grow. It distances water molecules, causing them to be more energetic and subsequently more reactive. This allows the plant cell to stretch, grow, and divide. Magnesium is also important for chlorophyll synthesis, which is necessary for photosynthesis.
Signs of deficiency: Magnesium deficiency in plants typically causes yellowing of leaf margins, interveinal chlorosis, and interveinal blotching. As magnesium deficiency progresses, leaves wither and die, plant growth is stunted, and blooming is limited or completely halted.
Sulfur (S)

Where it comes from: Almost all sulfur on Earth is present in the form of various compounds. However, there are two primary sources of sulfur that plants rely on for their production – sulfate ions are introduced into the environment by volcanic eruptions or by atmospheric release of hydrogen sulfide from decaying organic matter.
How it’s absorbed: In plants, sulfur is absorbed from the soil by the roots and transported to the leaves. Sulfur combines with iron and magnesium to form various proteins and ATPase (a protein that is found in all plant cells and helps cells produce energy).
Why it’s important: Sulfur helps your plants produce seeds, as well as promote root growth. It is also essential as a nutrient for chlorophyll, which is necessary for photosynthesis.
Signs of deficiency: Sulfur nutrient deficiency symptoms are very similar to those of nitrogen deficiency. Similar in that, symptoms are stunted growth and leaf curling. Although dissimilar in that, the entire leaves will sometimes turn a pale green rather than a yellow color.

share this image on your site
Micronutrients for plants
Although required in small quantities, micronutrients are still essential for plant health.
Even with the essential elements that are covered by the secondary nutrients and macronutrients, without the proper nutrients that are needed at a smaller scale, plants won’t grow adequately and may not have the resources needed to reproduce.
Boron (B)

Where it comes from: Boron predominantly comes from rock weathering and decaying organic matter.
How it’s absorbed: Once in the soil, boron is absorbed by plant roots and taken up by leaf cells. Plant uptake of boron depends on its concentration in the rhizosphere (the area immediately surrounding the roots). When plants are deficient in boron, leaves will show abnormalities such as tip burn or chlorosis.
Why it’s important: For micronutrients, boron is one of the most important. It acts as a nutrient regulator and helps your plant make simple sugars and carbohydrates. It also promotes the growth of fruit and seeds. Also, plants need boron for cellular wall development and functioning.
Signs of deficiency: Symptoms of boron deficiency include brittle and yellowing leaves, as well as stunted plant growth. Boron deficiencies also cause deformities in the roots, shoots, leaves, and fruit.
Chlorine (Cl)

Where it comes from: The largest concentration of chlorine on Earth exists as free chlorine gas. These are present in the atmosphere and combine with other elements to create salt. Chlorine is predominantly deposited in soil by rainwater that has formed from evaporating salt water.
How it’s absorbed: Specialized plant roots absorb chlorine from deposits from within the soil.
Why it’s important: Chlorine is an important element in the production of glucose, which is a key ingredient for photosynthesis. It is necessary to break down organic compounds, which are used by plants for energy. And it also helps with nitrogen fixation and protects against harmful pathogens such as bacteria and viruses.
Signs of deficiency: Chlorine deficiency is typically characterized by a lighter green color in leaves that may become curled inwards and yellowed. A chlorine deficient plant will typically exhibit a more narrow leaf shape with a pointed tip, a lower leaf surface area, and often smaller petioles.
Chlorine deficiency can also lead to stunted or undersized growth.
Cobalt (Co)

Where it comes from: The most common natural source of cobalt is ore, found in the earth’s crust. Over time, with erosion and weathering, these deposits have made their way into soils by wind-blown dust and rainwater.
How it’s absorbed: Plants absorb Cobalt by taking in the mineral through root absorption. It then takes the mineral up to water vessels in order to distribute it throughout the stems and leaves of the plant.
Why it’s important: Cobalt is used in the chlorophyll molecule. It is an essential part of the reaction that takes place in photosynthesis, during which light energy is absorbed by the chlorophyll and passed to nearby molecules.
Signs of deficiency: Cobalt deficiency is difficult to diagnose because it often mimics other deficiencies. It can cause leaves to turn light green, eventually turning yellow.
Plants with cobalt deficiencies can also show signs of necrosis (death of plant tissue and cells). The roots may show some elongation and, as a result, the plant will often be stunted or smaller with fewer leaves and shorter internodes.
Copper (Cu)

Where it comes from: The copper that plants use is primarily recycled from the environment. Copper occurs naturally in rocks and minerals, to which it binds weakly. This means that when these rocks and minerals are weathered by the environment (via erosion or other processes), their copper can be released into water and soil, where it becomes available to plant life.
How it’s absorbed: Through their root system, plants absorb copper from the soil or by absorbing dissolved copper from water.
Why it’s important: Copper helps with photosynthesis and aids in the production of chlorophyll. Copper is also required for some important functions in plants, such as phytochelatin – a copper-containing enzyme that binds to heavy metals and toxins. This acts to remove these impurities, detoxifying the plant.
Signs of deficiency: In plants, copper deficiency is shown by a variety of symptoms that range from necrotic lesions or chlorosis that develops first on the edges of the leaves. Symptoms include a lack of growth and a wilted appearance. The leaves may be twisted and curled as well as have a brownish tint. There can also be an overall loss of vitality in the plant.
Manganese (Mn)

Where it comes from: Manganese rich substances such as granite come from volcanic eruptions or magma that make their way to the surface. Once there, erosion breaks down these deposits and eventually leaches the element into the soil – ready for plant uptake.
How it’s absorbed: Plants absorb manganese through their root system, drawing manganese ions from the soil. As water is drawn to the surface via osmosis, so are these ions.
Why it’s important: Manganese is an important micronutrient for plants. Manganese is involved in antioxidant defense, iron metabolism, photosynthesis, nitrogen fixation, and chlorophyll formation.
Signs of deficiency: The most common symptoms of manganese deficiency in plants are interveinal chlorosis or yellowing of the leaves in between its veins. Other symptoms include chlorosis and necrosis of leaves, yellowing and mottling on the new growth tip, and decreased productivity.
Iron (Fe)

Where it comes from: Iron that is used by plants, or iron that has been absorbed from the soil, comes from a variety of sources. One of the most common sources is from complex organic molecules called chelates. These chelates bind to iron and carry it through the water of the plant’s cell wall to the rest of the plant.
How it’s absorbed: Iron is absorbed by plants through the roots and is transported throughout the plant via xylem (the vascular tissue that transports water and dissolved mineral nutrients upwards from a plant’s roots to its woody growth).
Why it’s important: Iron is one of the most important nutrients for plants. It is necessary for chlorophyll production, which is needed to convert light into energy. Iron also aids in the building of proteins and the storage of energy reserves. Plants also use iron to form vital enzymes such as cytochromes (a protein integral to cellular metabolism).
Signs of deficiency: Iron deficiency symptoms include poor leaf coloration and chlorosis, stunted development, reduced seed production, delayed flowering, and lack of fruit set.
Molybdenum (Mo)

Where it comes from: Most molybdenum is found in the earth’s crust as deposits left over by volcanic activity. These deposits over time have eroded and leached into the soil.
How it’s absorbed: Molybdenum is found in the soil, and like most soil borne nutrients, are absorbed through their roots.
Why it’s important: Molybdenum is an element that is vital to plant nutrition. It helps in the synthesis of plant proteins and DNA. It also aids in nitrate assimilation, ammonia detoxification, and the production of various enzymes.
Signs of deficiency: A plant deficient in molybdenum will not produce chlorophyll, which is what gives plants their green color. Consequently, the plant will become yellow or brownish-yellow with signs of stunted growth and eventual death.
Zinc (Zn)

Where it comes from: Zinc is one of the most abundant metals around. Although the most common natural source of zinc is leached rocks. This zinc is typically chelated with organic substances, such as carbonate or humus, which make it more bioavailable.
How it’s absorbed: Like other nutrients, this zinc is absorbed by roots and enters the xylem plant tissue to be distributed throughout its extremities.
Why it’s important: Zinc is useful in the synthesis of proteins and enzymes, which is vital for plant growth, reproduction, storage of energy, and defense against pathogens.
Signs of deficiency: Zinc deficiency in plants can show itself through a lack of coloration in the leaves. Plant leaves have a lower amount of chlorophyll, so they may appear yellow or white even if it is receiving plenty of light. In addition, zinc-deficient plants often cannot store proteins well, which will reduce their ability to resist insects and other pests.
Nickel (Ni)

Where it comes from: Plants use nickel that has been released to the environment by volcanic eruptions and natural weathering of Earth’s crust.
How it’s absorbed: Nickel, like other minerals in soil, is taken up by the roots and is then transported to various parts of the plant.
Why it’s important: Nickel is essential for plants to grow and photosynthesize, which is the process of converting carbon dioxide to sugars. Nickel also has a role in nitrogen fixation, which converts atmospheric nitrogen to ammonia.
Nickel also enhances plant immunity as it produces hydrogen peroxide, an antioxidant that helps fight off antibiotic-resistant bacteria.
Signs of deficiency: One of the most obvious signs of a nickel deficiency is the necrosis of a plant’s leaf tips because of toxic urea concentrations. Nickel deficiency is also shown as chlorosis in young leaves, a lack of dense and vigorous growth, and reduced leaf size.
What fertilizers we recommend
You may have heard people recommend sprinkling crushed eggshells, coffee grounds, or food scraps directly onto your plant’s soil as a good idea. However, these homemade soil amendments actually pose more risk to your houseplants than they do benefits.
Uncomposted food scraps or additives like coffee grounds attract fungus and bacteria, which can cause unwanted house plant infections and diseases.
This isn’t to say that food scraps aren’t worth repurposing into homemade plant fertilizer. I just recommend processing them through a bokashi bin, compost, or worm farm beforehand. This will not only eliminate the risk of houseplant infections and diseases but will also make the nutrients they contain more bioactive available to your indoor plants.
If you don’t have the option or simply don’t wish to create your bokashi juice, compost, or vermicompost for your houseplants, a better way to provide them with their primary nutrient requirements is to use an off-the-shelf fertilizer.
And if you really want to be super precise with your nutrient regime, a quality soil test kit will go a long way to determining exactly what primary and secondary nutrients your houseplants are begging you for.
Synthetic fertilizer options
Synthetic fertilizers are a great option as they are very reliable, their results are consistent, and the nutrients they contain are immediately bio actively available to plants.
Organic fertilizer options
Sure, organic fertilizers aren’t quite as reliable as their synthetic counterparts. It’s harder for manufacturers to accurately estimate the ratio of essential elements they contain, their results can vary, and often they are slower working as they are made from organic material, meaning they aren’t as readily absorbable as synthetically made elements.
Nevertheless, organic fertilizers are, after all, organic. And with a bit of time, a touch of patience, and the choice of a high-quality product, organic fertilizers can produce splendid results.
Key takeaway
Understanding the nutritional requirements of plants is key to creating an optimal environment for their health. Get this right and your houseplants will thank you by being fuller, healthier, and more colorful.
While you will thank yourself knowing that you are doing the best you can for your indoor garden.
Is there a more satisfying feeling than that?!
I don’t know of many.
Read our review of the best indoor plant fertilizers for more options.
More about fertilizing
- 10 Best Worm Composter Bins For Easy Homemade Compost
- Compost Starter 101: When You Need It And How To Make It
- Our top pothos fertilizer picks for luscious vines
- 5 reasons to use coffee as fertilizer for your plants
- Best fertilizer for Monstera plants for gorgeous leaves
- Fertilizer Burn on Plants? Here’s How to Fix it
- Fiddle leaf fig fertilizer: How to feed your fiddle leaf






